Pesonal iPS
Technology
Technology
We generate iPS cells from your urine or unneeded teeth and store them securely.
Would you like to create a new (potential) medical treatment for your child’s future?

Human iPS cells were first established in 2007 by Professor Shinya Yamanaka of Kyoto University. iPS cells are generally called “pluripotent cells” because they have the ability (pluripotency) to mature into any type of cell, such as heart, nerve, liver or blood cells.
iPS cells are currently being used worldwide for research centered on regenerative medicine. Regenerative medicine is a general term for medical treatment that aimes to renew or repair damaged cells by using transplanted cells, such as iPS cells. For example, research and clinical trials using iPS cells are being conducted with the aim of treating diseases such as Parkinson’s disease and AMD (age-related macular degeneration) which at present lack effective drugs and treatments. Regenerative medicine using iPS cells potentially offers a completely new treatment option to patients suffering from these diseases.
One of these clinical applications is to develop a method of generating iPS cells from the patient’s own cells and using for regenerative medicine such as transplantation surgery without rejection will be possible in the future.
Therefore, we offer a new system (service/concept) called “Personal iPS”.
How personal iPS works
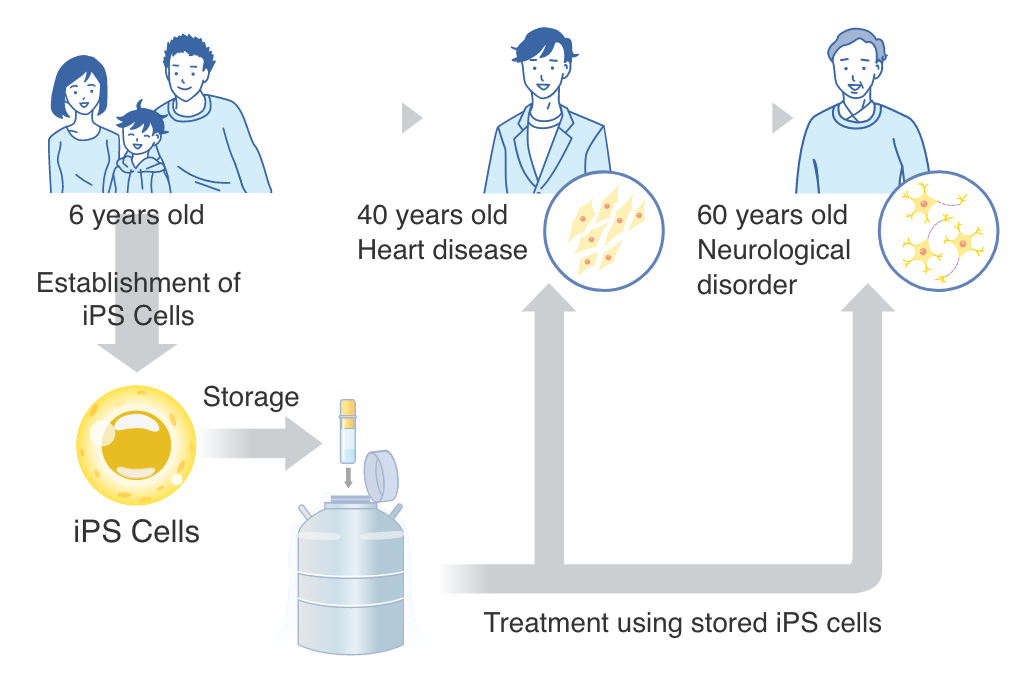
Personal iPS is a system that collects and stores your own cells before getting sick in the future
(before diseases strike you in the future.). Producing iPS cells in advance has the following advantages.
The younger you are, the fewer genetic mutations you have.
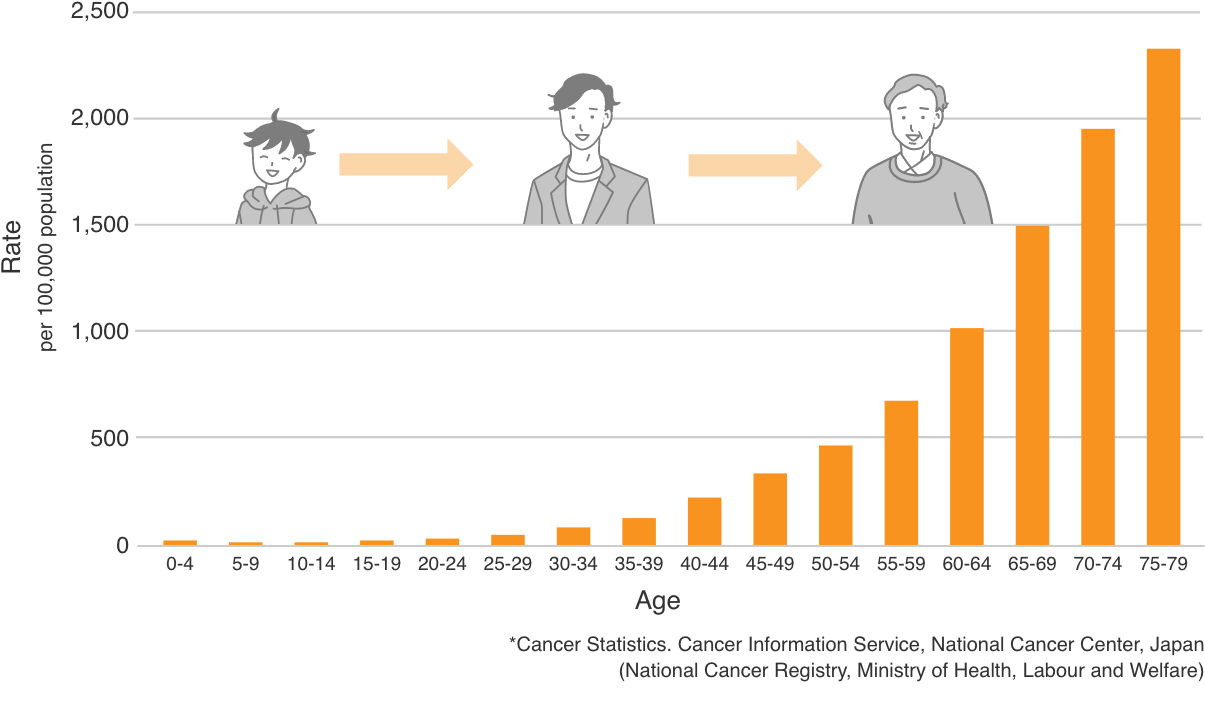
In general, it is said that the older we get, the more mutations are found in our genes, increasing the risk of cancer and other diseases. When the incidence of cancer is examined by age, statistics show that the risk of cancer increases with age. Generating iPS cells when young reduces the likely number of mutations in any cells stored for use later in life.
Sardo, V. L., Ferguson, W., Erikson, G. A., Topol, E. J., Baldwin, K. K., & Torkamani, A. (2017). The effect of aging on human induced pluripotent stem cells. Nature Biotechnology, 35(1), 69.
■Age Class Total Cancer Incidence Rate in Japan (2019)
Shortened time to treatment
Generally, it takes up to six months to produce iPS cells and assured the quality. Some injuries and diseases, such as spinal cord injuries and central nervous system diseases, have a better prognosis the earlier they are treated. Should treatment options with iPS cells become a reality, with our Personal iPSC service, we can be fully prepared in case of injuries or diseases and begin treatment as early as possible.

01

Development of iPS cells for various regenerative medicine
Part of the implementation status of clinical trials using iPS cells (as of April 2022)
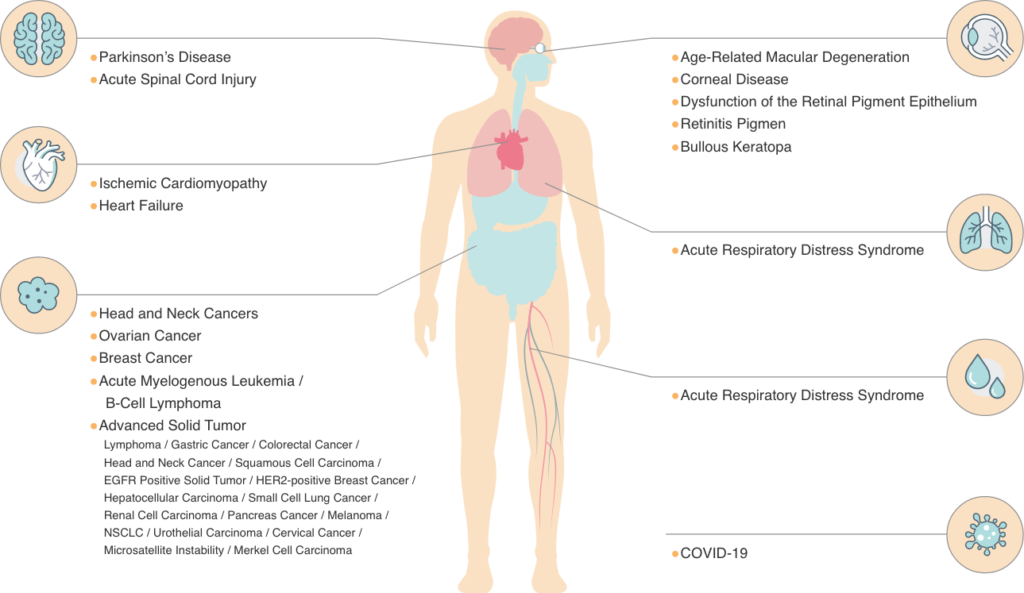
Since the invention of human iPS cells by Professor Shinya Yamanaka, iPS cells have been the subject of research and clinical trials worldwide. Recently, many practical research and development projects have been conducted, including the application of iPS cells to regenerative medicine. In Japan, clinical trials (including administration to humans) using iPS cells for AMD (age-related macular degeneration), Parkinson’s disease and heart disease have begun.
Age-related macular degeneration (AMD) is a medical condition that causes vision loss due to age-related damage to the macula, a tissue that plays an important role in vision. The macula is in the center of the retina where our eyes receive light and transmit signals to the brain through the optic nerve. The first clinical research worldwide on iPSC therapy for age-related macular degeneration was conducted at Kobe City Medical Center and RIKEN, and is still ongoing.
Parkinson’s disease (PD) is a progressive neurodegenerative disorder recognized by tremors in the hands and difficulty with walking. As Parkinson’s disease progresses, behavioral problems, depression and cognitive problems may also occur. People aged over 40 are more likely to develop the disease, with a particularly high percentage of those over the age of 65. Although there is no fundamental cure for Parkinson’s disease, clinical trials using iPS cells are underway at Kyoto University Hospital.
Ischemic heart disease (IHD) is a typical type of various heart diseases, it occurs when the blood vessels that carry oxygen and nutrients to the heart muscle (myocardium) become narrowed or blocked due to arteriosclerosis or other factors, preventing blood from reaching the myocardium.
The initial symptom is angina pectoris, which causes chest pain due to lack of blood supply to the myocardium, and in advanced stages, myocardial infarction, in which the blood vessels become completely clogged, leading to necrosis of the myocardium. The development of iPS cell-based therapies for cardiac diseases is underway at Osaka University and other companies.
Recently, clinical trials have commencedd for hematological cancers such as leukemia and lymphoma, and solid tumors such as ovarian cancer, head and neck cancer. Currently, lymphocytes (NK cells and T cells) with cancer cell-attacking properties differentiated from iPS cells are being tested in clinical trials targeting solid tumors led by pharmaceutical companies in particular in the U.S.
In Japan, where one out of every two people is said to have cancer, cancer therapy using iPS cells is an area that is expected to develop in the future.
As described above, the development of iPS cell-based therapies is advancing daily, and many are in the process of being administered to humans via clinical trials. The future of regenerative medicine using iPS cells is becoming a reality for many diseases that were once thought to be incurable.
02

Avoid risk of rejection
Personal iPS generates iPS cells from your own cells, thus rejection does not occur. In general, regenerative medicine can be divided into two categories, each of which has its own advantages.
- Autologous cell transplantation in which the patient’s own cells are harvested and transplanted back into the patient(individual)
- Cell transplantation from a healthy donor into the patient
| Advantage | Challenge | |
|---|---|---|
| Autologous Cell Transplantation | No rejection No donor search needed | Generally considered expensive, but Personal iPS achieves low cost |
| Allogeneic Cell Transplantation | Inexpensive Allow mass production | Rejection Difficult to find a donor |
The greatest advantage of autologous cell transplantation is that the treatment can be performed quickly, with no requirement to find a suitable donor, and because rejection does not occur, continuous immunosuppressive medications are not required. Some treatments have already been approved in Japan. Autologous cell transplantation is however often very expensive, as each treatment is manufactured on a customized basis for each patient.
The advantage of allogeneic treatment is that it can be mass-produced and provided at a relatively low cost. However, allogeneic treatment requires a donor with a HLA (tissue) type matched to the patient, making donor recruitment difficult and time-consuming. In addition, continuous immunosuppressive medication and checkups for rejection are required post transplantation.
Cases of current regenerative medicine application
Autologous treatments
Cancer Immunogen Therapy: Kymriah (U.S. pharmaceutical company)
Kymriah is a CAR T cell therapy for refractory B-cell acute lymphoblastic leukemia. White blood cells (T cells) are collected from the patient’s blood, transfected with genes to specifically attack the cancer, and then injected back into the patient’s body. Practical application is already underway in the U.S., and insurance coverage is available in Japan in 2019.
Spinal Cord Injury Therapeutics: Stemirac (Japanese pharmaceutical company)
Foreign companies are not the only providers of regenerative medicine products. This product for traumatic spinal cord injury treatment is the first one that has been approved worldwide. The treatment involves harvesting bone marrow cells from the spinal cord injury and then injecting them back after in vitro culturing.
Allogeneic treatments
Bone marrow transplant (public bank)
Bone marrow transplant is one of the treatments for leukemia. The bone marrow for transplantation is collected from donors registered with Japan Marrow Donor Bank and matches to the patient’s HLA (their tissue type).
Umbilical cord blood transplantation (public and private banks)
Umbilical cord blood is blood that remains in the placenta and in the attached umbilical cord after childbirth. Cord blood stored in public cord blood banks is used as one of the transplant treatments for leukemia.
Personal iPS produces iPS cells from your own, thus rejection does not occur. In addition, when it comes to the transplantation, it can be performed quickly without seeking for a donor. Furthermore, continuous immunosuppressive medications are not required that improves the quality of life.
Immune reaction when transplanting someone else’s cells
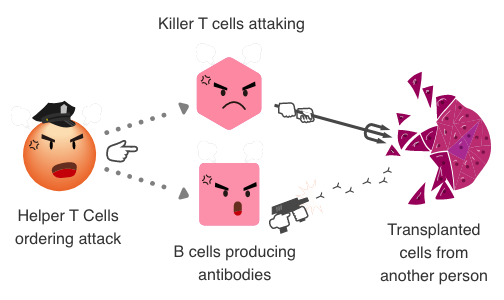
Immune response against own cells
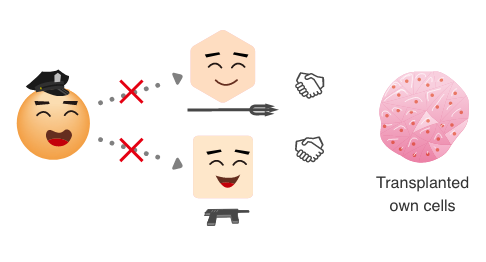
Our body is guarded by an immune system which detects and eliminates foreign substances (viruses, bacteria, etc.). When a foreign substance invades, helper T cells, one of the immune cells, detects it and directs their fellow killer T cells and B cells to attack and destroy the foreign substance.
Such immune responses do not occur with autologous cells and tissues.
03

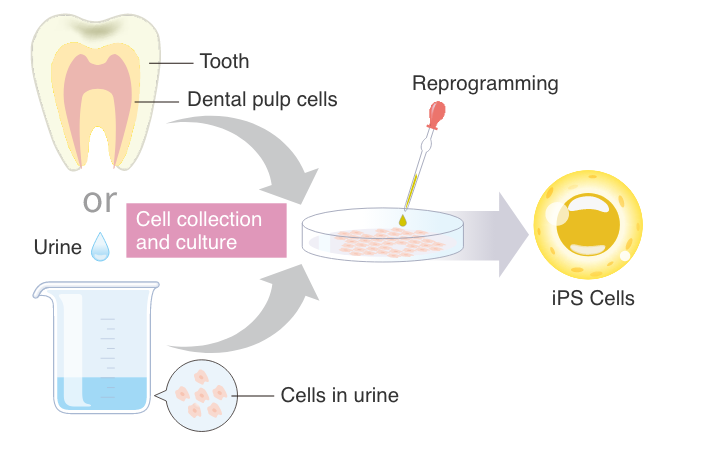
iPS cells derived from teeth and urine
Process of making iPS cells from unneeded teeth or urine
Generally, invasive methods such as skin biopsy or blood sampling are required for generating iPS cells.
Our non-invasive technology makes deriving iPS cells easy and for evryone by using unneeded teeth (e.g. from lost baby teeeth) and urine derived cells (UDCs).
The inside of the tooth and urine are basically sterile and contain dental pulp cells and UDCs, which are the source for generating iPS cells. After harvesting and expanding the teeth or urine cells, certain transcription factors are used to reprogram the adult cells into iPS cells, returing them to a state of “pluripotency” where they can in future be matured into any cell type.
*Reprogramming: Transformation of somatic cells into iPS cells.
04

Suitable iPS cells for regenerative medicine
Since the invention of iPS cells in 2007, the method of iPS cell production (reprogramming method) has been continuously improved. Originally, retroviral vector (foreign DNA from retroviruses) was used for reprogramming, however, there are risks of cancer development due to reactivation of foreign genes.
Besides the method above, there are three other reprogramming methods.
The first is the use of episomal vectors (foreign circular DNA). Since DNA is used as the transducing factor, it is difficult to completely rule out the possibility of integration of heritable viral DNA sequence into the host cell’s genome. *1
The second method, using Sendai virus vectors (foreign DNA derived from Sendai virus), avoids the risk of cancer development, but long-term persistence of foreign DNA has been reported. *2
The third is the “RNA reprogramming method”. The nature of RNA, non-integrating, and rapid degradation, makes it the most suitable and safe method to generate clinical grade iPS cells for regenerative medicine use currently. At REPROCELL, we have been conducting research and development of RNA reprogramming methods for many years and possess the most advanced technology.
RNA reprogramming method
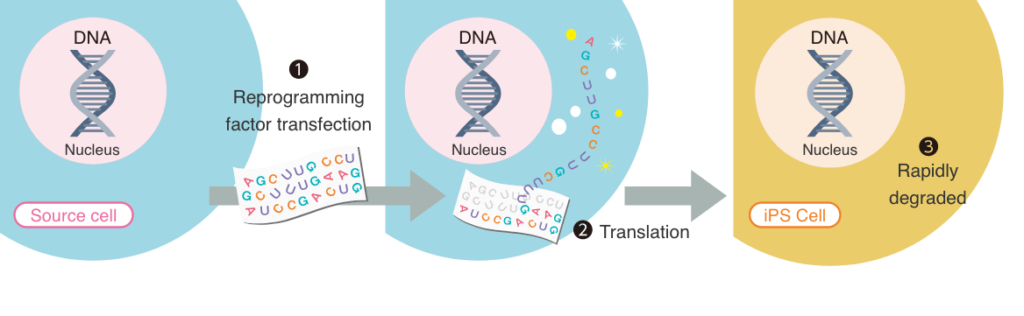
The figure above illustrates our method of producing iPS cells, the RNA reprogramming method. The nature of RNA: 1. they do not integrate into the genome; 2. they are degraded rapidly. Furthermore, it has been reported RNA delivery has the highest reprogramming efficiency compared to other methods. Thus, it is the most genomic integration free and footprint free method to generate clinical grade iPS cells.
*1: Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011 May;8(5):409-12.
*2: Agu CA, Soares FA, Alderton A, Patel M, Ansari R, Patel S, Forrest S, Yang F, Lineham J, Vallier L, Kirton CM. Successful Generation of Human Induced Pluripotent Stem Cell Lines from Blood Samples Held at Room Temperature for up to 48 hr. Stem Cell Reports. 20115 Oct 13;5(4):660-71.
05

Mass-produced iPS cells are stored at two locations in Japan and the U.S.
In Japan, typhoons and earthquakes happen frequently, the possibility that stored iPS cells may be at risk cannot be denied. For this reason, we store mass-produced iPS cells at two locations, in Japan and the U.S., in case of emergency, to ensure the protection of our customers’ iPS cells.
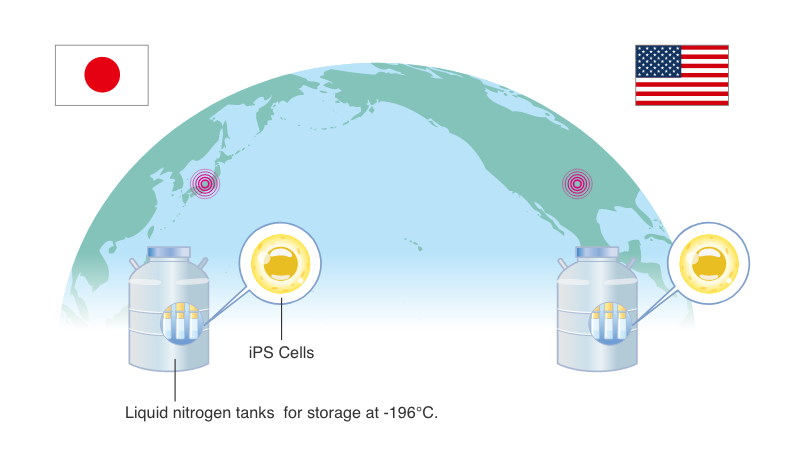
About the U.S. Office(branch)
Our U.S. location is a biobank with 30 years of experience
Establishment: 1989
Location: Maryland (U.S.)
Number of samples held: More than 500,000 specimens
Certification: CLIA (No. 21D1059245)
Partner Institutions: Fox Chase Cancer Center
Client: More than 1,000 institutions, including major pharmaceutical companies
06

Leading company of iPS cell
Since our establishment in 2003, we have been engaged in research and development of iPS cells for decades. In the process, we have harnesses cutting-edge technologies from our worldwide research and can now offer a Personal iPS service.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861-872.
● Osaka University
● Kyoto University
● Keio University
● University of Cambridge
● National Cancer Center Japan
● Juntendo University
● Durham University
● Tokai University
● Tokyo Institute of Technology
● Tokyo Women’s Medical University
● The University of Tokyo
● Massachusetts Institute of Technology
● Yokohama City University
Please use our Personal iPS service, available only from REPROCELL,
your trusted partner with a long track record of research and development of iPS cells.
